For the treatment of adult patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease
TRODELVY provided a statistically significant and clinically meaningful mPFS benefit vs chemotherapy1
Kaplan-Meier estimates of PFS by BICR based on RECIST 1.1 criteria (full population)1,2,*
*PFS is defined as the time from the date of randomization to the date of the first radiological disease progression or death due to any cause, whichever comes first.1
3x longer mPFS vs single-agent chemotherapy2
Primary endpoint: Kaplan-Meier estimates of PFS by BICR based on RECIST 1.1 criteria (brain-met-negative population)2,†
- 88% of patients in the ASCENT study were brain-met–negative2
Exploratory findings in previously treated, stable brain-met–positive patients1
- mPFS was 2.8 months with TRODELVY (95% CI: 1.5–3.9) vs 1.6 months with single-agent chemotherapy (95% CI: 1.3–2.9); HR: 0.65 (95% CI: 0.35–1.22)
Reproduced with permission from Bardia et al, N Engl J Med, 2021; copyright Massachusetts Medical Society.
†PFS was defined as the time from the date of randomization to the date of the first radiological disease progression or death due to any cause, whichever comes first.1
In a post hoc subgroup analysis
mPFS of TRODELVY vs 4 single-agent chemotherapies in the comparator arm3,‡
Kaplan-Meier estimates of PFS by BICR based on RECIST 1.1 criteria (brain-met–negative population)3,‡,§
- 88% of patients in the ASCENT study were brain-met–negative, and PFS results of this subanalysis were consistent with the ASCENT primary findings2
‡Limitation: These results are from a post hoc subgroup analysis of the Phase 3 ASCENT study. The single-agent chemotherapy arms were not powered for statistical analysis or designed to compare against individual agents and should be considered descriptive only. Therefore, the results require cautious interpretation and could represent chance findings.2,4
§PFS was defined as the time from the date of randomization to the date of the first radiological disease progression or death due to any cause, whichever comes first.1
Select safety findings3:
- Key Grade ≥3 treatment-related adverse events (TRAEs) with TRODELVY vs eribulin included neutropenia (51% vs 31%), leukopenia (10% vs 5%), diarrhea (10% vs 0%), anemia (8% vs 2%), febrile neutropenia (6% vs 2%), fatigue (3% vs 5%), nausea (3% vs 1%), and vomiting (1% vs 1%)
- Key Grade ≥3 TRAEs with TRODELVY vs vinorelbine, capecitabine, and gemcitabine combined included neutropenia (51% vs 36%), leukopenia (10% vs 6%), diarrhea (10% vs 1%), anemia (8% vs 8%), febrile neutropenia (6% vs 2%), fatigue (3% vs 6%), nausea (3% vs 0%), and vomiting (1% vs 0%)
- Discontinuation rates due to treatment-emergent adverse events for TRODELVY, eribulin, vinorelbine, capecitabine, and gemcitabine were 5%, 2%, 10%, 7%, and 9%, respectively
- 1 treatment-related death was reported for the single-agent chemotherapy arm (eribulin; neutropenic sepsis) and none with TRODELVY
In a subgroup analysis of ASCENT
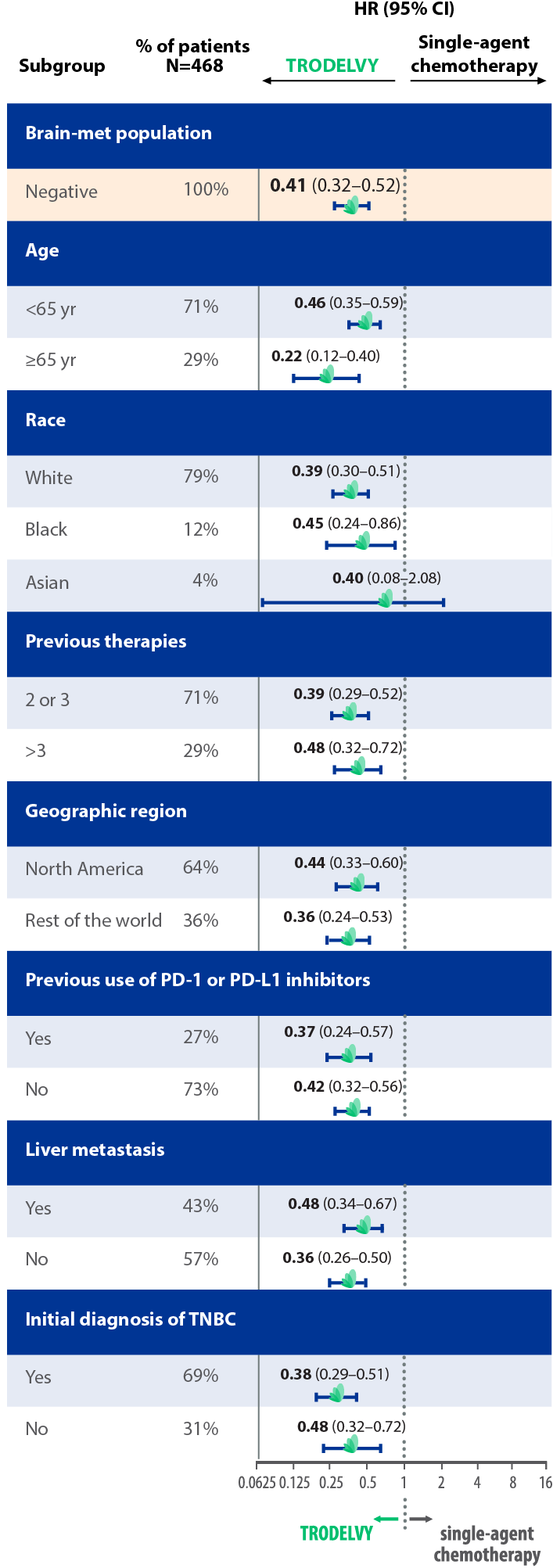
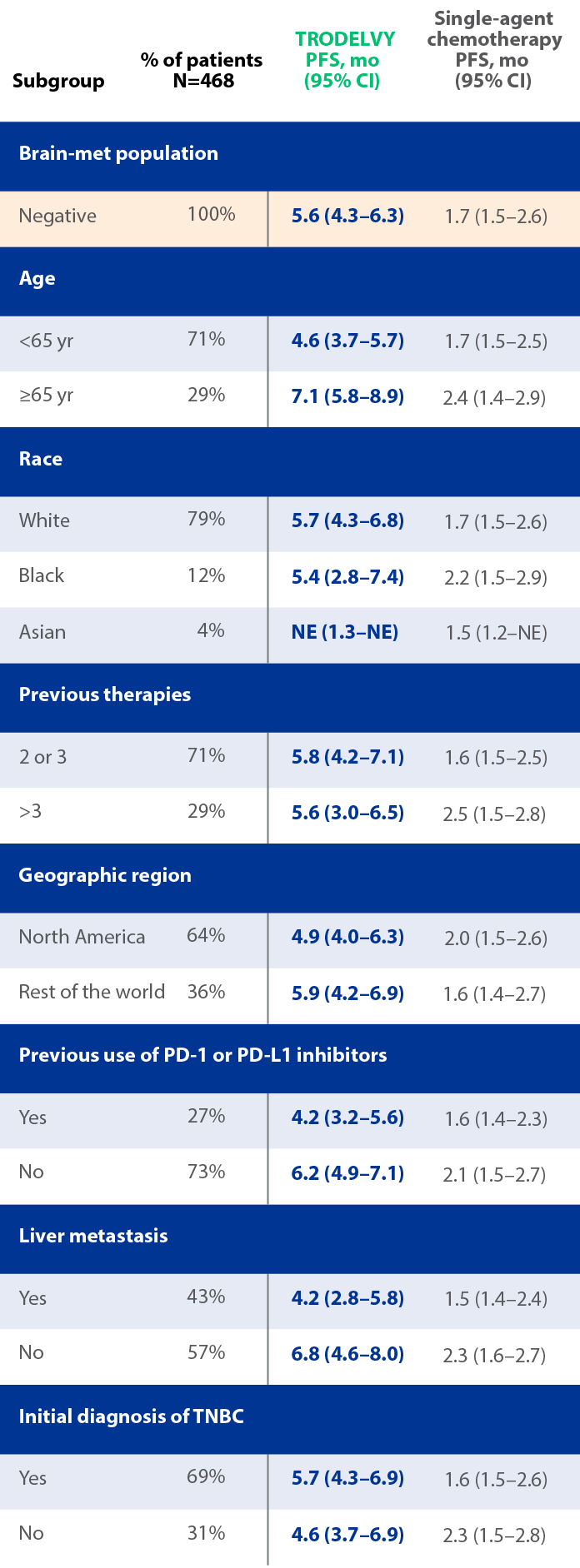
PFS results across subgroups in the primary analysis population1,||
||Limitation: These results are from a subgroup analysis of the Phase 3 ASCENT study. This secondary endpoint was not powered for statistical analysis and should be considered descriptive only. Therefore, the results require cautious interpretation and could represent chance findings.
mPFS by BICR based on RECIST 1.1 criteria and hazard ratio for disease progression or death (brain-met–negative population)2

- 88% of patients in the ASCENT study were brain-met–negative, and PFS results of this subanalysis were consistent with the ASCENT primary findings2
Reproduced with permission from Bardia et al, N Engl J Med, 2021; copyright Massachusetts Medical Society.
Discover TRODELVY in 2L and later mTNBC
with Yuan Yuan, MD, PhD
Director of Breast Oncology, Cedars-Sinai Cancer Center
2L=second line; BICR=blinded independent central review; brain-met=brain metastases; CI=confidence interval; mPFS=median progression-free survival; mTNBC=metastatic triple-negative breast cancer; PD-1=programmed cell death protein 1; PD-L1=programmed cell death ligand 1; PFS=progression-free survival; RECIST=Response Evaluation Criteria in Solid Tumors; TRAE=treatment-related adverse event; yr=year.
References: 1. TRODELVY. Prescribing Information. Gilead Sciences, Inc.; March 2025. 2. Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529-1541. 3. O’Shaughnessy J, Punie K, Oliveira M, et al. Assessment of sacituzumab govitecan vs treatment of physician’s choice cohort by agent in the phase 3 ASCENT study of patients with metastatic triple-negative breast cancer. Poster presented at: American Society of Clinical Oncology Annual Meeting; June 4-8, 2021. Poster 1077. https://meetings.asco.org/meetings/2021-asco-annual-meeting/273/program-guide/scheduled-sessions 4. Hurvitz SA, Bardia A, Punie K, et al. Sacituzumab govitecan efficacy in patients with metastatic triple-negative breast cancer by HER2 immunohistochemistry status: findings from the phase 3 ASCENT study. Poster presented at: European Society for Medical Oncology Breast Cancer Congress; May 3-5, 2022; Berlin, Germany. Poster 168P.
TRODELVY® (sacituzumab govitecan-hziy) is a Trop-2-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of adult patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
- TRODELVY can cause severe, life-threatening, or fatal neutropenia. Withhold TRODELVY for absolute neutrophil count below 1500/mm3 or neutropenic fever. Monitor blood cell counts periodically during treatment. Primary prophylaxis with G-CSF is recommended for all patients at increased risk of febrile neutropenia. Initiate anti-infective treatment in patients with febrile neutropenia without delay.
- TRODELVY can cause severe diarrhea. Monitor patients with diarrhea and give fluid and electrolytes as needed. At the onset of diarrhea, evaluate for infectious causes and, if negative, promptly initiate loperamide. If severe diarrhea occurs, withhold TRODELVY until resolved to ≤Grade 1 and reduce subsequent doses.
CONTRAINDICATIONS
- Severe hypersensitivity reaction to TRODELVY.
WARNINGS AND PRECAUTIONS
Neutropenia: Severe, life-threatening, or fatal neutropenia can occur as early as the first cycle of treatment and may require dose modification. Neutropenia occurred in 64% of patients treated with TRODELVY. Grade 3-4 neutropenia occurred in 49% of patients. Febrile neutropenia occurred in 6%. Neutropenic colitis occurred in 1.4%. Primary prophylaxis with G-CSF is recommended starting in the first cycle of treatment in all patients at increased risk of febrile neutropenia, including older patients, patients with previous neutropenia, poor performance status, organ dysfunction, or multiple comorbidities. Monitor absolute neutrophil count (ANC) during treatment. Withhold TRODELVY for ANC below 1500/mm3 on Day 1 of any cycle or below 1000/mm3 on Day 8 of any cycle. Withhold TRODELVY for neutropenic fever. Treat neutropenia with G-CSF and administer prophylaxis in subsequent cycles as clinically indicated or indicated in Table 2 of USPI.
Diarrhea: Diarrhea occurred in 64% of all patients treated with TRODELVY. Grade 3-4 diarrhea occurred in 11% of patients. One patient had intestinal perforation following diarrhea. Diarrhea that led to dehydration and subsequent acute kidney injury occurred in 0.7% of all patients. Withhold TRODELVY for Grade 3-4 diarrhea and resume when resolved to ≤Grade 1. At onset, evaluate for infectious causes and if negative, promptly initiate loperamide, 4 mg initially followed by 2 mg with every episode of diarrhea for a maximum of 16 mg daily. Discontinue loperamide 12 hours after diarrhea resolves. Additional supportive measures (e.g., fluid and electrolyte substitution) may also be employed as clinically indicated. Patients who exhibit an excessive cholinergic response to treatment can receive appropriate premedication (e.g., atropine) for subsequent treatments.
Hypersensitivity and Infusion-Related Reactions: TRODELVY can cause serious hypersensitivity reactions including life-threatening anaphylactic reactions. Severe signs and symptoms included cardiac arrest, hypotension, wheezing, angioedema, swelling, pneumonitis, and skin reactions. Hypersensitivity reactions within 24 hours of dosing occurred in 35% of patients. Grade 3-4 hypersensitivity occurred in 2% of patients. The incidence of hypersensitivity reactions leading to permanent discontinuation of TRODELVY was 0.2%. The incidence of anaphylactic reactions was 0.2%. Pre-infusion medication is recommended. Have medications and emergency equipment to treat such reactions available for immediate use. Observe patients closely for hypersensitivity and infusion-related reactions during each infusion and for at least 30 minutes after completion of each infusion. Permanently discontinue TRODELVY for Grade 4 infusion-related reactions.
Nausea and Vomiting: TRODELVY is emetogenic and can cause severe nausea and vomiting. Nausea occurred in 64% of all patients treated with TRODELVY and Grade 3-4 nausea occurred in 3% of these patients. Vomiting occurred in 35% of patients and Grade 3-4 vomiting occurred in 2% of these patients. Premedicate with a two or three drug combination regimen (e.g., dexamethasone with either a 5-HT3 receptor antagonist or an NK1 receptor antagonist as well as other drugs as indicated) for prevention of chemotherapy-induced nausea and vomiting (CINV). Withhold TRODELVY doses for Grade 3 nausea or Grade 3-4 vomiting and resume with additional supportive measures when resolved to Grade ≤1. Additional antiemetics and other supportive measures may also be employed as clinically indicated. All patients should be given take-home medications with clear instructions for prevention and treatment of nausea and vomiting.
Increased Risk of Adverse Reactions in Patients with Reduced UGT1A1 Activity: Patients homozygous for the uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1)*28 allele are at increased risk for neutropenia, febrile neutropenia, and anemia and may be at increased risk for other adverse reactions with TRODELVY. The incidence of Grade 3-4 neutropenia was 58% in patients homozygous for the UGT1A1*28, 49% in patients heterozygous for the UGT1A1*28 allele, and 43% in patients homozygous for the wild-type allele. The incidence of Grade 3-4 anemia was 21% in patients homozygous for the UGT1A1*28 allele, 10% in patients heterozygous for the UGT1A1*28 allele, and 9% in patients homozygous for the wild-type allele. Closely monitor patients with known reduced UGT1A1 activity for adverse reactions. Withhold or permanently discontinue TRODELVY based on clinical assessment of the onset, duration and severity of the observed adverse reactions in patients with evidence of acute early-onset or unusually severe adverse reactions, which may indicate reduced UGT1A1 function.
Embryo-Fetal Toxicity: Based on its mechanism of action, TRODELVY can cause teratogenicity and/or embryo-fetal lethality when administered to a pregnant woman. TRODELVY contains a genotoxic component, SN-38, and targets rapidly dividing cells. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TRODELVY and for 6 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TRODELVY and for 3 months after the last dose.
ADVERSE REACTIONS
In the pooled safety population, the most common (≥25%) adverse reactions including laboratory abnormalities were decreased leukocyte count (84%), decreased neutrophil count (75%), decreased hemoglobin (69%), diarrhea (64%), nausea (64%), decreased lymphocyte count (63%), fatigue (51%), alopecia (45%), constipation (37%), increased glucose (37%), decreased albumin (35%), vomiting (35%), decreased appetite (30%), decreased creatinine clearance (28%), increased alkaline phosphatase (28%), decreased magnesium (27%), decreased potassium (26%), and decreased sodium (26%).
In the ASCENT study, the most common adverse reactions (incidence ≥25%) were fatigue, diarrhea, nausea, alopecia, constipation, vomiting, abdominal pain, and decreased appetite. The most frequent serious adverse reactions (SAR) (>1%) were neutropenia (7%), diarrhea (4%), and pneumonia (3%). SAR were reported in 27% of patients, and 5% discontinued therapy due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the ASCENT study were reduced neutrophils, leukocytes, and lymphocytes.
DRUG INTERACTIONS
UGT1A1 Inhibitors: Concomitant administration of TRODELVY with inhibitors of UGT1A1 may increase the incidence of adverse reactions due to potential increase in systemic exposure to SN-38. Avoid administering UGT1A1 inhibitors with TRODELVY.
UGT1A1 Inducers: Exposure to SN-38 may be reduced in patients concomitantly receiving UGT1A1 enzyme inducers. Avoid administering UGT1A1 inducers with TRODELVY.
Please see full Prescribing Information, including BOXED WARNING.